Frequently Asked Questions
About Luveris®
Luveris (lutropin alfa for injection) is a fertility medication that contains luteinizing hormone (LH), which belongs to a group of hormones called “gonadotropins.” They are produced by the pituitary gland in the brain and play a role in the reproductive cycle and fertility.
Luveris is used to help eggs develop in women (patients with ovaries) undergoing procedures to help them become pregnant, such as in vitro fertilization (IVF). It is usually given along with a medication containing follicle-stimulating hormone (FSH).
About Luveris vials and syringes
Luveris comes as a dry powder. An additional vial containing sterile water is also provided. You will need to mix them to prepare the medication just before use. Each vial is intended for single use only.
Luveris is available at a strength of 75 IU.
“IU” stands for International Units.
- Store vials between 2-25°C (either in the refrigerator or at room temperature)
- Protect from light
- Do not expose to extreme heat or cold
- Do not use after the expiry date indicated on the label
- Do not use Luveris if:
- you notice any signs of deterioration or damage to the container
-
- the solution contains particles or is not clear
Once you have finished your injection, immediately dispose of the needles and syringe (without recapping the needle) into a sharps container. This container is usually provided by your clinic or pharmacy.
Taking Luveris
To feel more comfortable giving yourself an injection of Luveris, you can:
- Watch the Luveris injection video
- Download a step-by-step injection guide to follow as you administer Luveris
- Call our toll-free helpline for one-on-one injection assistance from registered nurses at 1-800-387-8479
For complete injection instructions, refer to the package insert that came with your Luveris.
Luveris is given by subcutaneous injection (just under the skin). You can learn to do your own injections in the comfort and privacy of your own home.
The easiest places to give yourself a Luveris injection are your abdomen and thighs. You may find the injection is more comfortable if you use a different injection site each day.
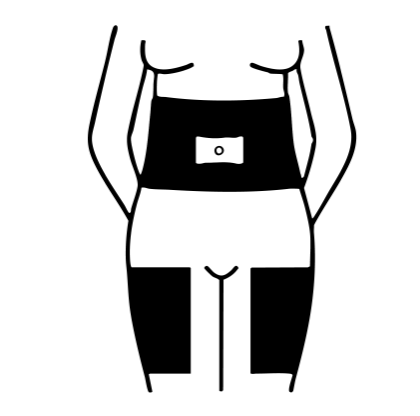
Shaded areas show recommended injection sites
Contact your doctor for advice if you forget to take a dose of Luveris. Do not take a double dose to make up for any doses you have missed.
If you have accidentally injected too much Luveris, contact your clinic or another healthcare professional, hospital emergency department, or regional Poison Control Centre immediately, even if there are no symptoms.
Safety and side effects
Do not use Luveris if you:
- Are allergic (hypersensitive) to gonadotropins (such as luteinizing hormone, follicle-stimulating hormone, or human chorionic gonadotropin), or any of the other ingredients of Luveris
- Have been diagnosed with cancer of the ovary, uterus, or breast
- Have been diagnosed with a brain tumour
- Have large ovaries or sacs of fluid (ovarian cyst) not due to polycystic ovarian disease (PCOS) and of unknown origin
- Have unexplained vaginal bleeding
Luveris must not be used when a condition exists which would make a spontaneous pregnancy impossible, such as:
- Premature menopause
- Malformation of sexual organs
- Specific tumours of the womb
Before you use Luveris, talk to your doctor or pharmacist if you:
- Have sex hormone–dependent tumours of the reproductive tract and accessory organs
- Have active, untreated tumours of the hypothalamus or pituitary gland
- Are pregnant or are breastfeeding (feeding a baby your own milk)
- Have ovarian failure
- Have abnormal uterine bleeding of unknown origin
- Are allergic to gonadotropins or to any of the non-medicinal ingredients
Other warnings you should know about:
- Reproductive issues: Incidence of reproductive issues is increased compared with natural conception; these include ectopic pregnancy (a pregnancy that occurs outside of the uterus) in patients with a history of disease or scarring of the fallopian tubes, miscarriage, or being pregnant with more than one child (i.e., a multiple pregnancy, usually twins)
- Tumours of the ovary and other reproductive organs: Both benign and malignant tumours have been reported in patients who have undergone multiple drug regimens for treatment of infertility
- Thromboembolic events: Treatment with gonadotropins, and even pregnancy itself, can increase the risk of thromboembolic events (formation of a blood clot in a vein or artery) in individuals at higher risk due to personal or family history
- Congenital abnormalities: There have been unconfirmed findings of higher rates of congenital abnormalities in babies conceived through assisted reproduction techniques (ART) compared to those conceived naturally
Refer to the Luveris package insert for information.
As with any medication, you may experience side effects when taking Luveris. The most common side effects include:
- Headache
- Pelvic and abdominal pain
- Nausea
- Ovarian hyperstimulation syndrome (OHSS)
- Breast pain/pain in the soft tissues of chest/underarm
- Ovarian cysts
- Flatulence (passing gas)
- Injection site reactions
- General pain
- Constipation
- Fatigue
- Painful menstruation
- Ovarian disorder
- Diarrhea
- Upper respiratory tract infections
When taking Luveris, there is a risk of developing OHSS. Since OHSS develops rapidly, contact your doctor if you experience any early warning signs of OHSS, including:
- Severe abdominal pain
- Nausea
- Vomiting
- Weight gain
This is not a complete list of side effects. Refer to the package insert for more information about possible side effects with Luveris, including serious side effects.
If you experience any unusual symptoms or side effects while taking Luveris, report them to your doctor or pharmacist immediately.
No significant drug interactions have been reported with Luveris. However, you should still tell your doctor or pharmacist about all the medicines you are currently taking or have recently taken, including any over-the-counter medicines.
Have more questions about Luveris?
Call Momentum toll-free at 1-800-387-8479