
Frequently Asked Questions
About OVIDREL®
OVIDREL (choriogonadotropin alfa) is a fertility medication that contains human chorionic gonadotropin (hCG). hCG is a hormone that is involved in the female reproductive cycle.
OVIDREL is used to trigger ovulation (the release of eggs from the ovaries) following the stimulation phase of fertility treatment.
About the OVIDREL pre-filled pen
OVIDREL comes as a liquid formulation (250 μg/0.5 mL) in a pre-filled, ready-to-use pen.
- Store between 2-8°C (in a refrigerator)
- Do not freeze
- Store in the original package
- Protect from light
- Do not use the OVIDREL pre-filled pen if the solution contains particles or is not clear
- Keep out of reach of children
- Do not use after the expiry date
You should dispose of the used needle safely in a sharps container. This container is usually provided by your clinic or pharmacy.
You can dispose of your used OVIDREL Pens in an environmentally responsible manner by participating in the EMD Serono ecoprogram. Download our ecoprogram brochure to learn more.
Taking OVIDREL
To feel more comfortable giving yourself an injection of OVIDREL, you can:
- Watch the OVIDREL injection video
- Download a step-by-step injection guide to follow as you administer OVIDREL
- Call our toll-free helpline for one-on-one injection assistance from registered nurses at 1-800-387-8479
For complete injection instructions, refer to the package insert that came with your OVIDREL Pen.
OVIDREL is given by subcutaneous injection (just under the skin).
OVIDREL should be injected in your abdomen or thigh.
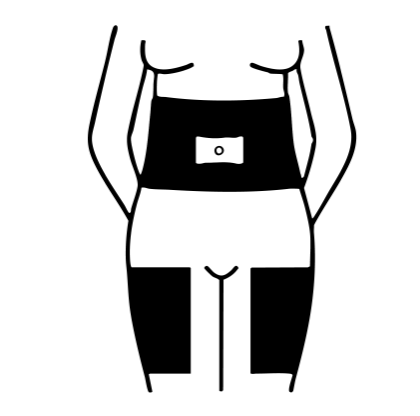
Shaded areas show recommended injection sites
Safety and side effects
Do not use OVIDREL if you:
- Are allergic to choriogonadotropin alfa or to any of the other ingredients of OVIDREL
- Have a tumour in your hypothalamus or pituitary gland (both are parts of the brain)
- Have large ovaries or sacs of fluid within the ovaries (ovarian cysts) of unknown origin
- Have unexplained vaginal bleeding
- Have cancer of your ovaries, womb, or breast
- Have severe inflammation of your veins or blood clotting in your veins (active thromboembolic disorders)
- Have any condition that usually makes a normal pregnancy impossible, such as menopause or early menopause (ovarian failure), or malformations of sexual organs
- Have an uncontrolled thyroid or adrenal function
- Are currently pregnant
To help avoid side effects and ensure proper use, talk to your doctor or pharmacist before you take OVIDREL.
Using OVIDREL may raise your risk of:
- Blood clot
- Ovarian hyperstimulation syndrome (OHSS): While uncommon (less than 3% of patients), it can progress rapidly to a serious medical event
- Symptoms include:
- Severe pain or bloating in the stomach or pelvic area
- Severe upset stomach
- Vomiting
- Weight gain
- OVIDREL should be stopped at the first signs of OHSS; contact your doctor if you experience these symptoms
- Symptoms include:
Other warnings you should know about:
- Reproductive issues: Fertility treatments are also associated with risk of reproductive issues including miscarriage, ectopic pregnancy (a pregnancy that occurs outside of the uterus), or being pregnant with more than one child (i.e., a multiple pregnancy)
Refer to the OVIDREL package insert for more information about these warnings.
As with all medications, side effects can occur with the use of OVIDREL. Side effects that have been reported include:
- Discomfort at the injection site
- Stomach pain
- Nausea
- Vomiting
Ask your fertility team to discuss the possible side effects with you, and refer to the package insert for more information about possible side effects with OVIDREL. As with all medications, it is important to report any physical changes and all symptoms to your doctor.
Please inform your doctor and pharmacist if you are taking or have taken any other medications, including over-the-counter drugs, vitamins, minerals, natural supplements, or alternative medicines.
Have more questions about OVIDREL?
Call Momentum toll-free at 1-800-387-8479